The Accidental Invention of Stainless Steel: A Rustless Revolution
Why Do We Turn to Stainless Steel?
Iron, a metal known for its strength and versatility, has one significant drawback: it rusts. Exposure to water and heat causes iron to corrode, diminishing its durability and aesthetic appeal. This problem led to a relentless search for a rust-resistant alternative.
Harry Brearley
In 1913, Harry Brearley, a metallurgist from Sheffield, England, embarked on an experiment to find a solution.
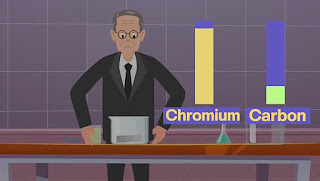
He began by mixing iron with various other metals, but his early attempts were met with failure. However, Brearley didn't give up. After several trials and extensive research, he stumbled upon a material that didn't corrode. Curious about this discovery, he analyzed it further and found that the key to its rust resistance was the high chromium content combined with low carbon levels.
To confirm his findings, Brearley conducted additional experiments to see how this new material reacted with chemicals. His tests revealed that not only was this metal rust-resistant, but it also had other remarkable properties. Brearley shared his discovery with a friend, who tested the material by adding vinegar. Remarkably, the metal remained unscathed, confirming its rustless nature.
This breakthrough led to the invention of what we now know as stainless steel—a material that has become indispensable in industries worldwide. Its ability to resist corrosion while maintaining strength has made it a preferred choice for everything from kitchen utensils to skyscrapers.








No comments:
Post a Comment